RESEARCH
Low-dose Stress Group
We have understood well how cells are killed by high-level or lethal stresses at the molecular level.
In contrast, we do not know much about the consequences of subtle but constantly occurring environmental changes. It is difficult to decipher how such low-dose stress impacts the life history of organisms since they often do not elicit any obvious phenotypic changes, such as cell death.
In contrast, we do not know much about the consequences of subtle but constantly occurring environmental changes. It is difficult to decipher how such low-dose stress impacts the life history of organisms since they often do not elicit any obvious phenotypic changes, such as cell death.
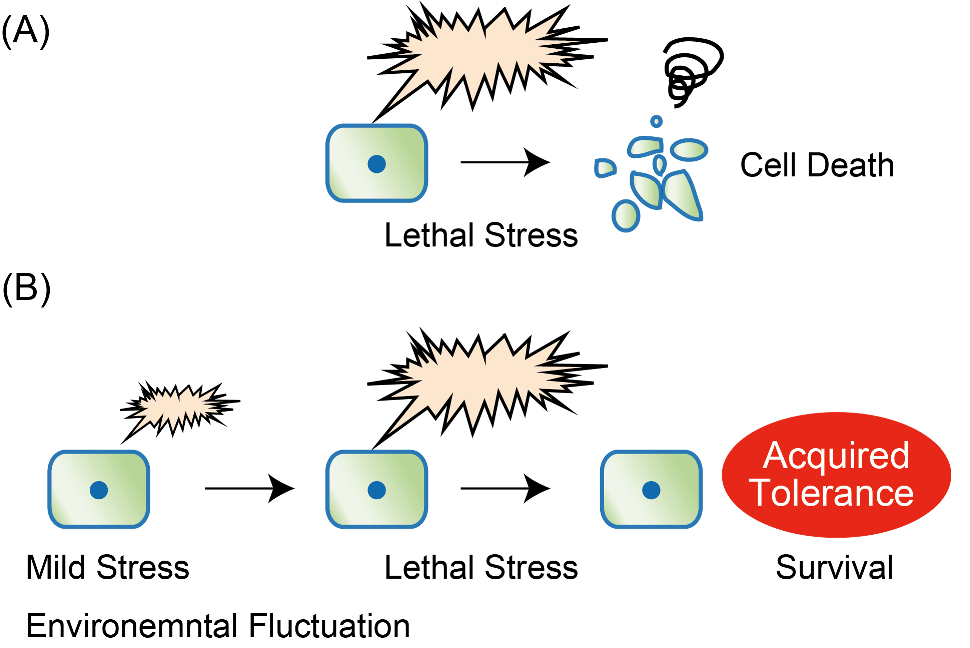
In this regard, Prof. Ishikawa is particularly interested in hormesis. Hormesis refers to the phenomena where exposure to a mild stress causes adaptively beneficial effects on an organism's viability and reproduction (fecundity), while exposure to a strong dose of the same type of stress causes organismal death. We have investigated the molecular mechanisms of this non-intuitive and intriguing biological process. Normal cells and tissues in multi-cellular organisms cooperate with each other to maintain the metabolism. In contrast, cancer cells do not enjoy any support from the surrounding normal tissues. This is because a cancer cell's goal is to proliferate as efficiently as possible, by constantly changing its genome via mutations. The resultant cancer genome governs new physiological rules and strategies, distinct from those of normal cells - making the cooperation between cancer and normal cells impossible. Blood supply to cancer cells is scarce at least in the early phase of cancer development, for example. Arguably, cancer cells are experiencing mild but continuous stress posed by their surrounding normal cells in their niche. According to the theory of hormesis, this mild stress may confer cancer cells resistance to prospective strong stresses, such as iatrogenic anti-cancer therapy. We expect understanding the biochemical mechanisms of hormesis may eventually lead to development of novel of anti-cancer treatments.